Industrial ammonia production is very energy intensive and has a huge carbon dioxide footprint. A plasma integrated water electrolyser provides an ecologically clean alternative process to Haber-Bosch without carbon dioxide emissions, when using green electrical power.
Designing an all-electric process: plasma-nitrogen fixation on a water electrolyser
The main goal of the project was to develop a plasma integrated water electrolyser towards proof of concept phase, which is schematically depicted in Figure 1. Herein, the main challenge of electrochemical nitrogen fixation is the competing reaction to ammonia synthesis: the production of hydrogen.
Plasma nitrogen activation was examined as a parameter to tune the selectivity towards ammonia synthesis. Furthermore, process efficiency estimation and catalyst efficiency screening were performed, to provide a perspective for plasma aided ammonia synthesis.
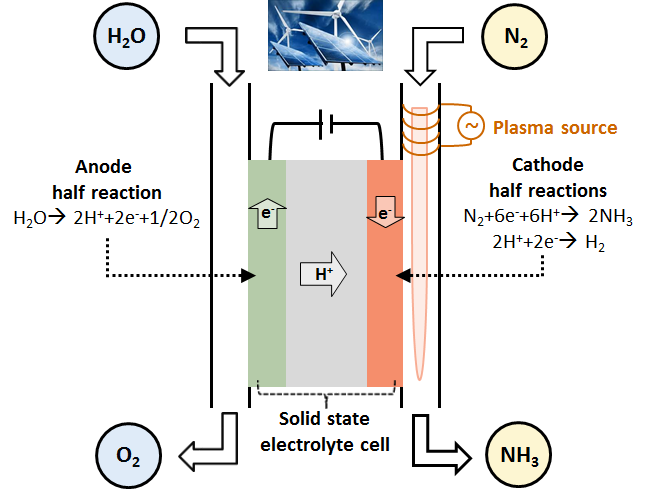
Plasma assisted electrochemical nitrogen fixation for nitric oxide and ammonia synthesis from N2 and H2O is encouraging, since a record high faradaic efficiency and production rate have been achieved for both NO (93%) and NH3 (88%). In addition, this approach will be very helpful in improving the understanding of plasma-catalytic mechanisms, due to the absence of byproducts and need of co-activation of O2/H2 with N2.
Successful development of an integrated plasma-aided electrochemical device
An integrated plasma aided electrochemical device was successfully developed (Figure 2), the approach was published in scientific peer-reviewed journals. Please find the publication overview below.
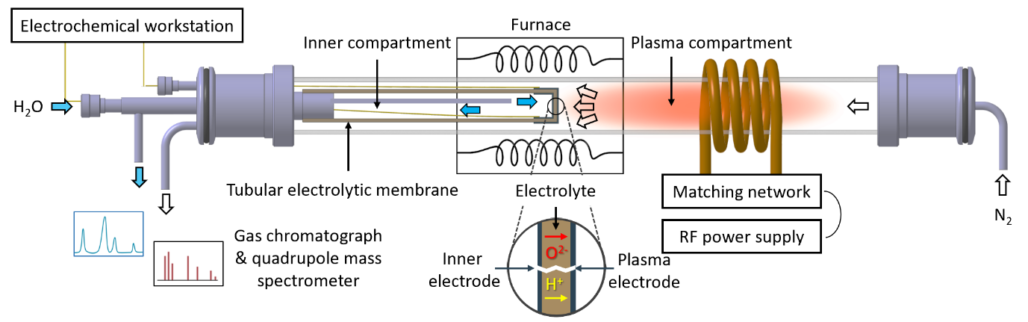
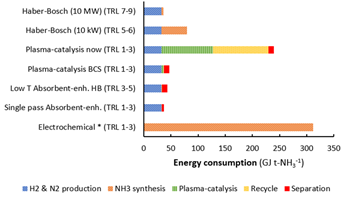
Taking a process perspective, many key operating process parameters need to be evaluated such as temperature, flow rate, distance between the solid oxide electrolyser cell and active plasma as well as electrode materials and its architecture. From the process evaluation for plasma aided ammonia synthesis, it follows that significant steps of improvements need to be realized in order to achieve a feasible plasma driven route. Even in the best case scenario (BCS), the energy consumption for plasma-catalytic NH3 synthesis is higher than for an absorbent-enhanced Haber-Bosch process (Figure 3), making plasma aided ammonia synthesis unlikely at the industrial scale.
A different focus for future projects
Instead, further research on small-scale ammonia synthesis is better focused on ammonia separation under mild conditions with, for instance, solid sorbents. Plasma aided NOX synthesis shows better potential, due to the potential lower energy consumption and the lower capital expenditure as compared to the conventional processes. Therefore, future research should focus on enhancing the production rate and lowering the energy consumption for plasma aided NOX synthesis.
Please find all project result details in the final report, available here.
You might also be interested in
Acknowledgement & partners
This project is co-funded by TKI-E&I with the supplementary grant 'TKI- Toeslag' for Topconsortia for Knowledge and Innovation (TKI’s) of the Ministry of Economic Affairs and Climate Policy.









