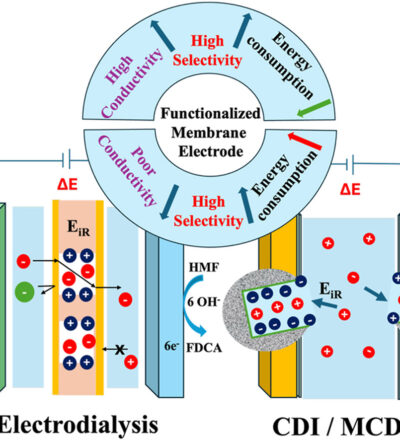
Electrodialysis (ED) and capacitive deionization (CDI) are electrically driven separation technologies with strong potential to advance renewable and sustainable goals, as they can be powered by renewable electricity, while enabling water purification and recovery of valuable chemicals. Thus, minimizing energy consumption greatly enhances the sustainable operation of these devices. In this review, we systematically analyze various sources of energy losses in ED and CDI and evaluate how advances in ion exchange membranes, and electrodes affect these losses. We have characterized the energy losses based on the minimum voltage required to overcome interfacial barriers at the electrode–electrolyte and membrane–solution interfaces. We further examine how membrane modification strategies influence the area-specific resistance and specific energy consumption (SEC) in ED, while the membrane coated electrode thickness governs SEC in CDI. Our comparative analysis reveals that ohmic losses
have a greater impact in CDI due to surface-limited salt adsorption, whereas ED performance is strongly dictated by the membrane resistance. Additionally, we highlight the integration of paired electrochemical reactions, such as CO2 reduction to formic acid (~0.2–0.5 V vs. SHE), as sustainable alternatives to water splitting (~1.23 V), enabling simultaneous separation and value-added chemical synthesis in a single device. Collectively, this review provides a framework for identifying material-level strategies to minimize energy losses, reduce SEC and enhance the sustainability of next-generation ED and CDI systems. These insights highlight pathways towards renewable powered, low energy separation processes that support global clean water and sustainable chemical production goals.
Download your free copy
Our publications are free to access. Simply provide your first name and email address to download.